Lippy
GUDID 00681490669771
PROSTHESIS 1133265 LIPPY CUP PSTN .6X5.5
MEDTRONIC XOMED, INC.
Ossicular prosthesis, total Ossicular prosthesis, total Ossicular prosthesis, total Ossicular prosthesis, total Ossicular prosthesis, total Ossicular prosthesis, total Ossicular prosthesis, total Ossicular prosthesis, total Ossicular prosthesis, total Ossicular prosthesis, total Ossicular prosthesis, total Ossicular prosthesis, total Ossicular prosthesis, total Ossicular prosthesis, total Ossicular prosthesis, total Ossicular prosthesis, total Ossicular prosthesis, total Ossicular prosthesis, total Ossicular prosthesis, total Ossicular prosthesis, total Ossicular prosthesis, total Ossicular prosthesis, total Ossicular prosthesis, total Ossicular prosthesis, total Ossicular prosthesis, total Ossicular prosthesis, total| Primary Device ID | 00681490669771 |
| NIH Device Record Key | f91aa7aa-0e5b-494e-b75c-d24dd6595409 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Lippy |
| Version Model Number | 1133265 |
| Company DUNS | 835465063 |
| Company Name | MEDTRONIC XOMED, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | MR Conditional |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
Device Dimensions
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 5.5 Millimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 5.5 Millimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 5.5 Millimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 5.5 Millimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 5.5 Millimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 5.5 Millimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 5.5 Millimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 5.5 Millimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 5.5 Millimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 5.5 Millimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 5.5 Millimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 5.5 Millimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 5.5 Millimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 5.5 Millimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 5.5 Millimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 5.5 Millimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 5.5 Millimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 5.5 Millimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 5.5 Millimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 5.5 Millimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 5.5 Millimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 5.5 Millimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 5.5 Millimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Length | 5.5 Millimeter |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00681490669771 [Primary] |
FDA Product Code
| ETA | REPLACEMENT, OSSICULAR PROSTHESIS, TOTAL |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 2 |
| Public Version Date | 2018-03-29 |
| Device Publish Date | 2015-06-23 |
On-Brand Devices [Lippy]
| 00681490669771 | PROSTHESIS 1133265 LIPPY CUP PSTN .6X5.5 |
| 00681490034685 | PROSTHESIS 1133300 LIPPY CUP PSTN .4X6.0 |
| 00681490034678 | PROSTHESIS 1133295 LIPPY CUP PSTN .4X5.5 |
| 00681490034661 | PROSTHESIS 1133290 LIPPY CUP PSTN .4X5.0 |
| 00681490034654 | PROSTHESIS 1133285 LIPPY CUP PSTN .4X4.5 |
| 00681490034647 | PROSTHESIS 1133280 LIPPY CUP PSTN .4X4.0 |
| 00681490034630 | PROSTHESIS 1133270 LIPPY CUP PSTN .6X6.0 |
| 00681490034623 | PROSTHESIS 1133260 LIPPY CUP PSTN .6X5.0 |
| 00681490034616 | PROSTHESIS 1133255 LIPPY CUP PSTN .6X4.5 |
| 00681490034609 | PROSTHESIS 1133250 LIPPY CUP PSTN .6X4.0 |
Trademark Results [Lippy]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
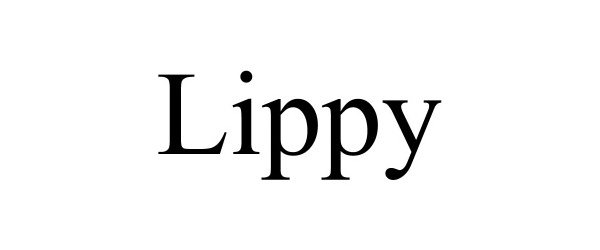 LIPPY 90326128 not registered Live/Pending |
Amron, David 2020-11-18 |
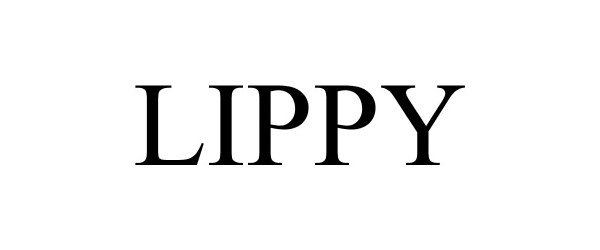 LIPPY 88618356 not registered Live/Pending |
LIPPY LLC 2019-09-16 |
 LIPPY 78305207 not registered Dead/Abandoned |
998232 Alberta Ltd. 2003-09-25 |
 LIPPY 78305203 not registered Dead/Abandoned |
998232 Alberta Ltd. 2003-09-25 |
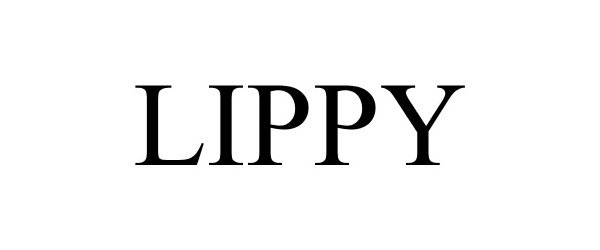 LIPPY 77710238 not registered Dead/Abandoned |
Robert P. Nickell 2009-04-09 |
 LIPPY 73477465 1323316 Dead/Cancelled |
Pretty Industries, Inc. 1984-04-26 |
© 2024 FDA.report
This site is not affiliated with or endorsed by the FDA.