ESPEC
GUDID 00802807356782
SINGLE READER
Evolutioneyes, Inc.
Magnifying spectacles Magnifying spectacles Magnifying spectacles Magnifying spectacles Magnifying spectacles Magnifying spectacles Magnifying spectacles Magnifying spectacles Magnifying spectacles Magnifying spectacles Magnifying spectacles Magnifying spectacles Magnifying spectacles Magnifying spectacles Magnifying spectacles Magnifying spectacles| Primary Device ID | 00802807356782 |
| NIH Device Record Key | 57dd563c-a7b0-412d-8fa3-bc908575869c |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | ESPEC |
| Version Model Number | 4345 CS MAG |
| Company DUNS | 825224459 |
| Company Name | Evolutioneyes, Inc. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00802807356782 [Primary] |
FDA Product Code
| HOI | Spectacle, magnifying |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2024-05-08 |
| Device Publish Date | 2024-04-30 |
On-Brand Devices [ESPEC]
| 00802807354306 | SINGLE READER |
| 00802807354221 | SINGLE READER |
| 00802807354214 | SINGLE READER |
| 00802807354122 | SINGLE READER |
| 00802807354115 | SINGLE READER |
| 00802807354108 | SINGLE READER |
| 00802807354092 | SINGLE READER |
| 00802807353750 | SINGLE READER |
| 00802807353743 | SINGLE READER |
| 00802807354801 | SINGLE READER |
| 00802807354825 | SINGLE READER |
| 00802807354818 | SINGLE READER |
| 00802807355099 | SINGLE READER |
| 00802807355129 | SINGLE READER |
| 00802807355297 | SINGLE READER |
| 00802807356263 | SINGLE READER |
| 00802807356331 | SINGLE READER |
| 00802807356157 | SINGLE READER |
| 00802807356058 | SINGLE READER |
| 00802807356126 | SINGLE READER |
| 00802807356720 | SINGLE READER |
| 00802807356928 | SINGLE READER |
| 00802807356782 | SINGLE READER |
Trademark Results [ESPEC]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ESPEC 90144534 not registered Live/Pending |
DNOW L.P. 2020-08-28 |
 ESPEC 76378322 3117486 Live/Registered |
Espec Kabushiki Kaisha 2002-03-04 |
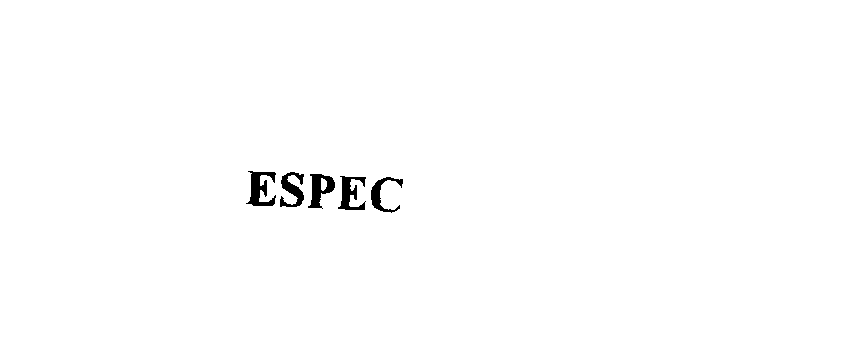 ESPEC 76052572 not registered Dead/Abandoned |
ThunderPointe.com, Inc 2000-05-19 |
 ESPEC 75672170 not registered Dead/Abandoned |
ESPEC CORP. 1999-03-31 |
 ESPEC 73426169 1358900 Live/Registered |
Tabai Espec Kabushiki Kaisha 1983-05-16 |
© 2024 FDA.report
This site is not affiliated with or endorsed by the FDA.