MBlock 05.05.0090
GUDID 03760304680144
The MBlock device consists of an adjustable nonabsorbable braided loop and titanium button, preloaded with traction threads, designed to be used for ACL reconstruction. The implant is supplied sterile, ready to use.
S.B.M
Tendon/ligament bone anchor, non-bioabsorbable Tendon/ligament bone anchor, non-bioabsorbable Tendon/ligament bone anchor, non-bioabsorbable Tendon/ligament bone anchor, non-bioabsorbable Tendon/ligament bone anchor, non-bioabsorbable Tendon/ligament bone anchor, non-bioabsorbable Tendon/ligament bone anchor, non-bioabsorbable Tendon/ligament bone anchor, non-bioabsorbable Tendon/ligament bone anchor, non-bioabsorbable Tendon/ligament bone anchor, non-bioabsorbable Tendon/ligament bone anchor, non-bioabsorbable Tendon/ligament bone anchor, non-bioabsorbable Tendon/ligament bone anchor, non-bioabsorbable Tendon/ligament bone anchor, non-bioabsorbable Tendon/ligament bone anchor, non-bioabsorbable Tendon/ligament bone anchor, non-bioabsorbable Tendon/ligament bone anchor, non-bioabsorbable| Primary Device ID | 03760304680144 |
| NIH Device Record Key | 6b7dd1c5-d39b-4676-b3ea-2e534d9a63ed |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | MBlock |
| Version Model Number | Adjustable Loop XL |
| Catalog Number | 05.05.0090 |
| Company DUNS | 772462123 |
| Company Name | S.B.M |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | 901-203-3470 |
| clussier@medacta.us.com | |
| Phone | 901-203-3470 |
| clussier@medacta.us.com | |
| Phone | 901-203-3470 |
| clussier@medacta.us.com | |
| Phone | 901-203-3470 |
| clussier@medacta.us.com | |
| Phone | 901-203-3470 |
| clussier@medacta.us.com | |
| Phone | 901-203-3470 |
| clussier@medacta.us.com | |
| Phone | 901-203-3470 |
| clussier@medacta.us.com | |
| Phone | 901-203-3470 |
| clussier@medacta.us.com | |
| Phone | 901-203-3470 |
| clussier@medacta.us.com | |
| Phone | 901-203-3470 |
| clussier@medacta.us.com | |
| Phone | 901-203-3470 |
| clussier@medacta.us.com | |
| Phone | 901-203-3470 |
| clussier@medacta.us.com | |
| Phone | 901-203-3470 |
| clussier@medacta.us.com | |
| Phone | 901-203-3470 |
| clussier@medacta.us.com | |
| Phone | 901-203-3470 |
| clussier@medacta.us.com | |
| Phone | 901-203-3470 |
| clussier@medacta.us.com | |
| Phone | 901-203-3470 |
| clussier@medacta.us.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 03760304680144 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K151004
FDA Product Code
| MBI | Fastener, Fixation, Nondegradable, Soft Tissue |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 2 |
| Public Version Date | 2023-08-14 |
| Device Publish Date | 2020-11-16 |
On-Brand Devices [MBlock]
| 03760304680168 | The MBlock device consists of an adjustable nonabsorbable braided loop and titanium button, prel |
| 03760304680151 | The MBlock device consists of an adjustable nonabsorbable braided loop and titanium button, prel |
| 03760304680144 | The MBlock device consists of an adjustable nonabsorbable braided loop and titanium button, prel |
| 03760304680137 | The MBlock device consists of an adjustable nonabsorbable braided loop and titanium button, prel |
Trademark Results [MBlock]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
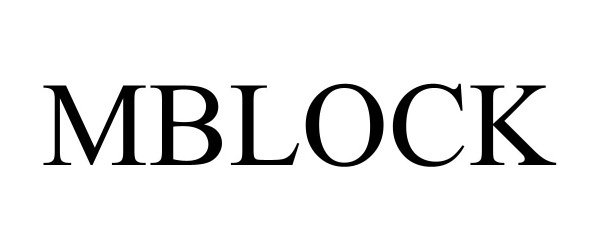 MBLOCK 97674617 not registered Live/Pending |
SWITCH PROJECT, LLC 2022-11-12 |
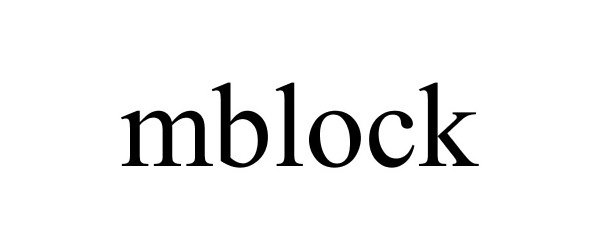 MBLOCK 87703984 not registered Live/Pending |
MAKEBLOCK CO., LTD. 2017-11-30 |
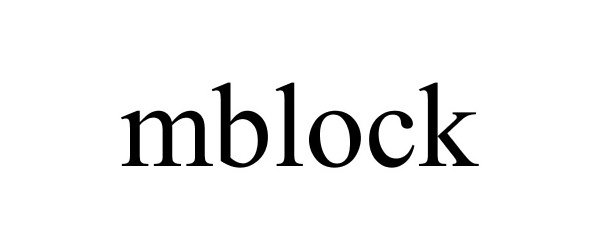 MBLOCK 87703983 not registered Live/Pending |
MAKEBLOCK CO., LTD. 2017-11-30 |
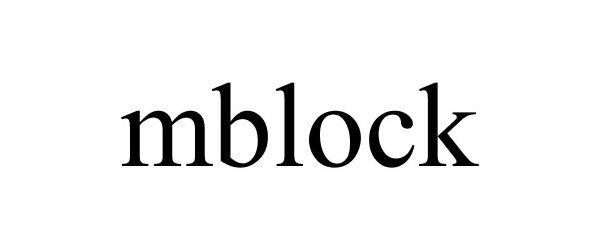 MBLOCK 87703982 not registered Live/Pending |
MAKEBLOCK CO., LTD. 2017-11-30 |
© 2024 FDA.report
This site is not affiliated with or endorsed by the FDA.