Praxiject™ 0.9% NaCl 3705-1US
GUDID 20682686004547
10 mL of 0.9% Sodium Chloride Injection USP in a 10 mL syringe
Medxl Inc
Vascular catheter/cannula flush solution, non-anticoagulant, non-antimicrobial Vascular catheter/cannula flush solution, non-anticoagulant, non-antimicrobial Vascular catheter/cannula flush solution, non-anticoagulant, non-antimicrobial Vascular catheter/cannula flush solution, non-anticoagulant, non-antimicrobial Vascular catheter/cannula flush solution, non-anticoagulant, non-antimicrobial Vascular catheter/cannula flush solution, non-anticoagulant, non-antimicrobial Vascular catheter/cannula flush solution, non-anticoagulant, non-antimicrobial Vascular catheter/cannula flush solution, non-anticoagulant, non-antimicrobial Vascular catheter/cannula flush solution, non-anticoagulant, non-antimicrobial Vascular catheter/cannula flush solution, non-anticoagulant, non-antimicrobial Vascular catheter/cannula flush solution, non-anticoagulant, non-antimicrobial| Primary Device ID | 20682686004547 |
| NIH Device Record Key | 419f2ac8-2e92-4988-b920-ffcd1d7f4932 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Praxiject™ 0.9% NaCl |
| Version Model Number | 3705-1US |
| Catalog Number | 3705-1US |
| Company DUNS | 259901445 |
| Company Name | Medxl Inc |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1.514.695.7474 |
| sales@medxl.com | |
| Phone | +1.514.695.7474 |
| sales@medxl.com | |
| Phone | +1.514.695.7474 |
| sales@medxl.com | |
| Phone | +1.514.695.7474 |
| sales@medxl.com | |
| Phone | +1.514.695.7474 |
| sales@medxl.com | |
| Phone | +1.514.695.7474 |
| sales@medxl.com | |
| Phone | +1.514.695.7474 |
| sales@medxl.com | |
| Phone | +1.514.695.7474 |
| sales@medxl.com | |
| Phone | +1.514.695.7474 |
| sales@medxl.com | |
| Phone | +1.514.695.7474 |
| sales@medxl.com | |
| Phone | +1.514.695.7474 |
| sales@medxl.com |
Device Dimensions
| Total Volume | 10 Milliliter |
| Device Size Text, specify | 0 |
| Total Volume | 10 Milliliter |
| Device Size Text, specify | 0 |
| Total Volume | 10 Milliliter |
| Device Size Text, specify | 0 |
| Total Volume | 10 Milliliter |
| Device Size Text, specify | 0 |
| Total Volume | 10 Milliliter |
| Device Size Text, specify | 0 |
| Total Volume | 10 Milliliter |
| Device Size Text, specify | 0 |
| Total Volume | 10 Milliliter |
| Device Size Text, specify | 0 |
| Total Volume | 10 Milliliter |
| Device Size Text, specify | 0 |
| Total Volume | 10 Milliliter |
| Device Size Text, specify | 0 |
| Total Volume | 10 Milliliter |
| Device Size Text, specify | 0 |
| Total Volume | 10 Milliliter |
| Device Size Text, specify | 0 |
Operating and Storage Conditions
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 10682686004540 [Primary] |
| GS1 | 20682686004547 [Package] Contains: 10682686004540 Package: Carton [460 Units] In Commercial Distribution |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K192414
FDA Product Code
| NGT | Saline, Vascular Access Flush |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 2 |
| Public Version Date | 2024-05-07 |
| Device Publish Date | 2020-06-17 |
On-Brand Devices [Praxiject™ 0.9% NaCl]
| 30682686004513 | 5 mL of 0.9% Sodium Chloride Injection USP in a 10 mL syringe |
| 30682686004506 | 3 mL of 0.9% Sodium Chloride Injection USP in a 10 mL syringe |
| 30682686004490 | 10 mL of 0.9% Sodium Chloride Injection USP in a 10 mL syringe |
| 30682686004483 | 3 mL of 0.9% Sodium Chloride Injection USP in a 5 mL syringe |
| 10682686004472 | 5 mL of 0.9% Sodium Chloride Injection USP in a 5 mL syringe |
| 20682686004547 | 10 mL of 0.9% Sodium Chloride Injection USP in a 10 mL syringe |
Trademark Results [Praxiject]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
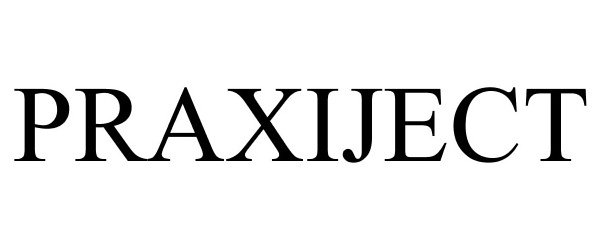 PRAXIJECT 87227552 5492995 Live/Registered |
MedXL Inc. 2016-11-05 |
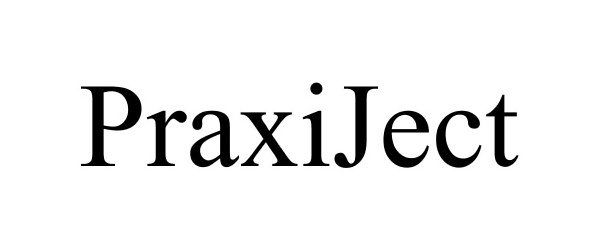 PRAXIJECT 77289384 3862391 Dead/Cancelled |
MedXL inc. 2007-09-26 |
© 2024 FDA.report
This site is not affiliated with or endorsed by the FDA.